India has taken a significant step forward in its battle against cervical cancer by introducing its first locally produced version of the human papillomavirus (HPV) vaccine, known as “Cervavac.” Currently, there is no national immunization program in India specifically targeting the eradication of cervical carcinoma. The incorporation of Cervavac into the national immunization schedule is expected to significantly enhance the fight against cervical cancer.
HPV constitutes a group of more than 200 related viruses, with sexually transmitted HPV types falling into two categories: low risk and high risk. High-risk HPVs can lead to various types of cancer. HPV infection is widespread, affecting nearly all sexually active individuals within months to a few years of becoming sexually active. While most HPV infections are non-cancerous and cleared by the immune system, approximately 1% of persistent high-risk HPV infections can progress to cancer. HPV infection is a well-established cause of cervical cancer, and there is growing evidence of its significance in other anogenital and head-and-neck cancers.
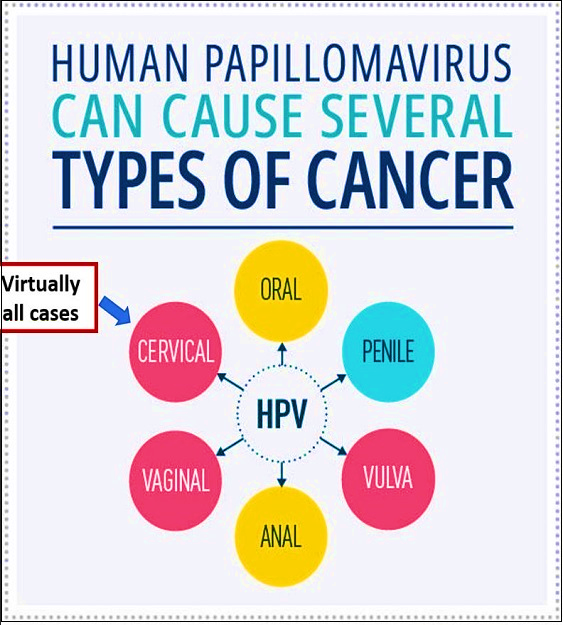
India, with a population of 511.4 million women aged 15 and older, faces a substantial risk of developing cervical cancer. Almost all cervical cancers are attributed to persistent infection with one of the 14 high-risk types of HPV, with HPV types 16 and 18 accounting for a significant percentage of cases globally and especially in India. Cervical cancer ranks as the second most prevalent cancer in India, with an estimated 1,23,907 new cases and 77,348 annual deaths, constituting close to one-fifth of the global burden. Unfortunately, more than three-quarters of cases are diagnosed at a locally advanced clinical stage with poor prospects of survival.
| 2008 | · Bivalent and quadrivalent vaccines licensed in India |
| 2009 | · HPV vaccination demonstration projects in Andhra Pradesh and Gujarat and seven deaths reported (Later found to be not associated with vaccination)· India IARC multicentre study on two versus three doses of HPV vaccination starts recruitment |
| 2010 | · HPV vaccination suspended in research studies but continues to be available for prescription |
| 2016 | · HPV vaccination initiated by Delhi State Government· Similar efficacy of two and three doses of HPV vaccination established in the India IARC study |
| 2017 | · Punjab initiates HPV vaccination in the Bathinda and Mansa districts |
| 2018 | · 2018 Similar efficacy of one versus two or three doses reported· Sikkim initiates state-wide HPV vaccination targeting girls aged 9-14 years in 1166 schools· Updated evidence from the India IARC study indicates two doses |
| 2019 | · Updated evidence from the India IARC study indicates two doses of the vaccine could be extended to girls aged 15-18 years |
| 2022 | · SII launched Cervavac, India’s first indigenously made HPV vaccine in September 2022 |
The history of the HPV vaccine’s implementation in India reflects various milestones, with the recent launch of Cervavac in September 2022 marking a significant development. The availability of an indigenous and more affordable vaccine like Cervavac could play a crucial role in addressing India’s cervical cancer burden, provided other barriers surrounding vaccination are simultaneously addressed. Inclusion in the national immunization program would increase awareness and acceptance, necessitating mass awareness campaigns to dispel vaccine-related myths, misconceptions, and stigma.
HPV vaccination provides safe, effective, and lasting protection against the HPV infections that most commonly lead to cancer. Recommendations for age and dosage, as per FOGSI GCPR/NCI/CDC guidelines, emphasize targeting the age group of 9-14 years with two doses administered at 0 and 6 months. Catch-up vaccination for those aged 15-26 involves three doses, while older age groups receive three doses with varying intervals. However, it’s crucial to note that HPV vaccination prevents new infections but does not treat existing HPV infections or diseases.
The World Health Organization (WHO) recommends updated schedules for HPV vaccination, including a one or two-dose schedule for girls aged 9-14, a one or two-dose schedule for girls and women aged 15-20, and two doses with a 6-month interval for women older than 21. Notably, the efficacy of a single dose with Cervavac is yet to be established.
Regarding vaccine safety, HPV vaccines are based on virus-like particles (VLPs) lacking the virus’s DNA, making them non-infectious. Over 15 years of monitoring has shown that the HPV vaccine is very safe, with minor side effects such as pain, redness, or swelling at the injection site, dizziness, or fainting, which is more common among adolescents.
Eliminating cervical cancer is now a feasible goal, with evidence-based tools available. In 2020, the World Health Organization approved a strategy aimed at global cervical cancer elimination. To achieve this, countries must reach and maintain an incidence rate of below 4 per 100,000 women. The strategy involves high vaccination rates, effective screening, and timely treatment.
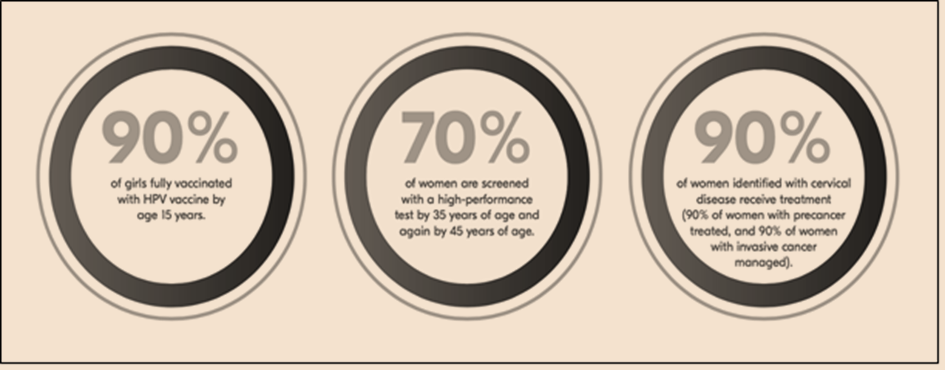
India, with its successful track record in childhood immunization and recent efforts in COVID-19 vaccination, stands to gain significantly from including the HPV vaccine in its national immunization schedule. Success requires strong political will, effective public awareness campaigns, and collaboration between government, NGOs, and other stakeholders. While improvements in screening, diagnosis, and treatment are urgently needed, widespread vaccination against human papillomavirus will have the most significant impact on eliminating the disease. The HPV vaccine serves as a crucial tool in preventing HPV-related cancers.








